Smooth lines, improve acne scars & more with SkinPen® Technology
Microneedling treatment that delivers maximum results
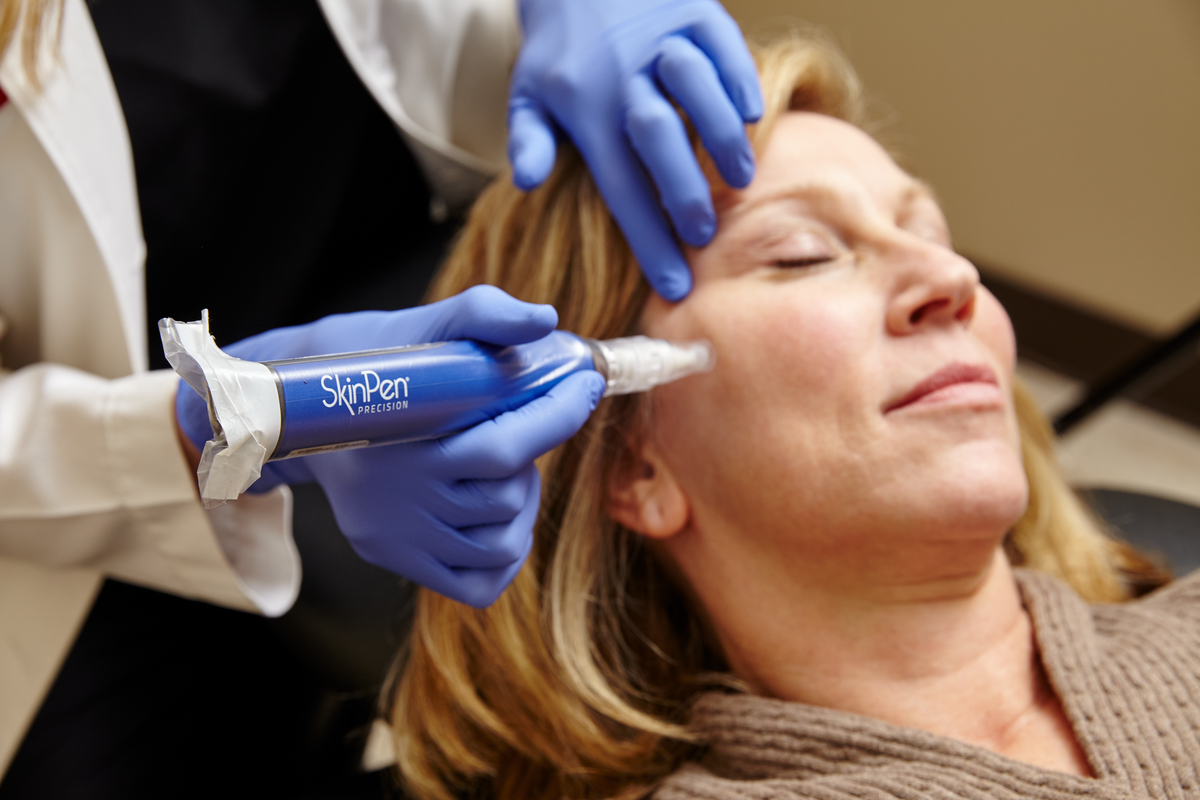
Microneedling is a cosmetic procedure in which fine needles are used to create tiny punctures in the skin, promoting skin rejuvenation and wound healing. It is used to treat fine lines and wrinkles, acne scars, and stretch marks. The procedure stimulates the production of collagen, a protein that helps to keep skin tight and firm, and can improve the overall texture and appearance of the skin.
At Amalurra Centre for Medical Aesthtics, our experts use the SkinPen® microneedling system to rejuvenate skin from the inside out, for youthful, refreshed look. SkinPen® works by activating your body’s natural skin-healing power to regenerate collagen and elastin production, without heat or chemicals. The treatment improves the look of wrinkles on the face and neck, acne scars, and your skin’s tone and texture. In as little as three 30-minute sessions, you can expect to see natural, transformative results, with little downtime and lasting positive effects.
Reserve your microneedling appointment today to reveal your best skin!
Slide title
Write your caption hereButton
Plus, you can combine your microneedling treatment with PRP Induction or Growth Factor to address scars, age/sun spots, large pores, discoloration, and improve your skin’s texture and vibrancy.
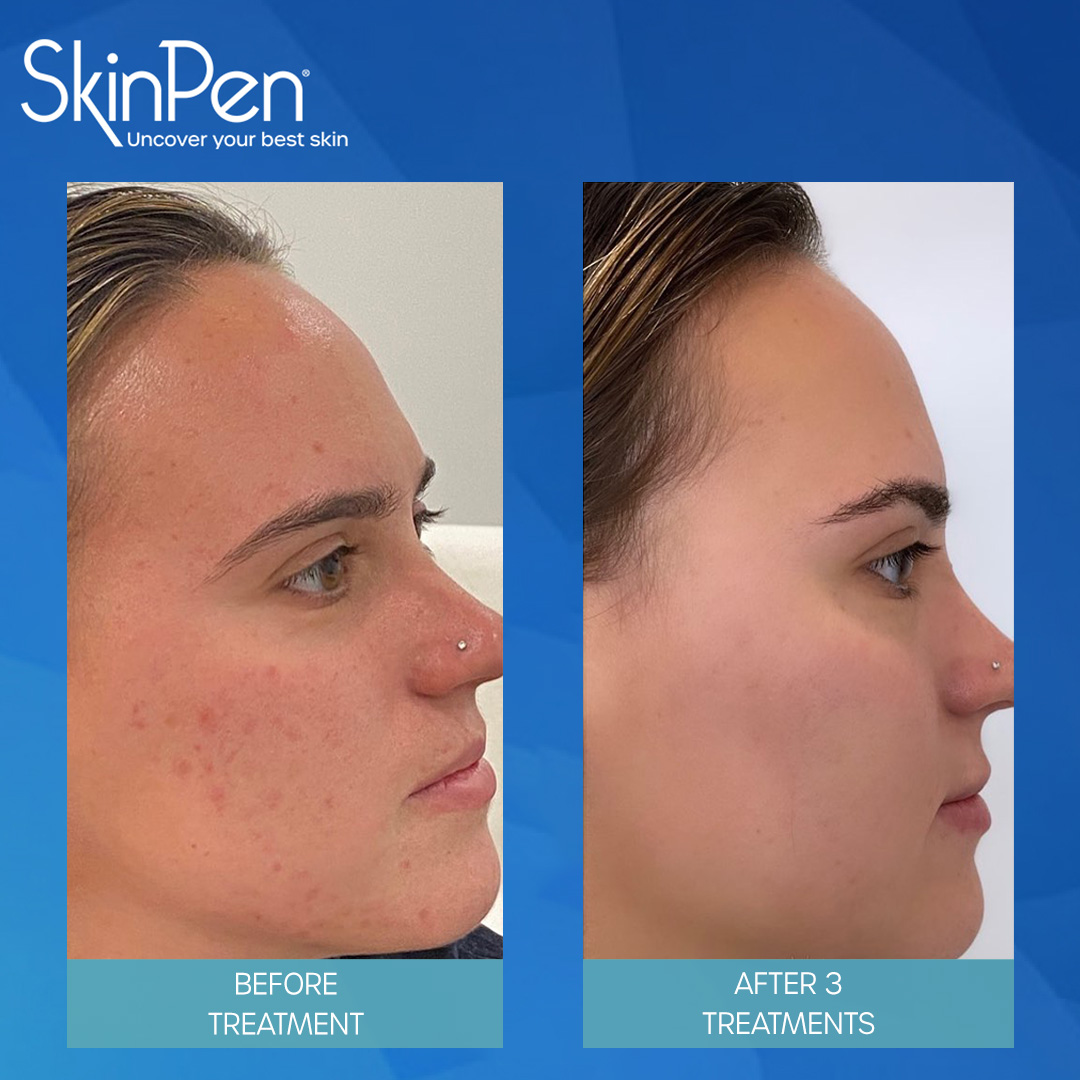
Real Patients before & after SkinPen® microneedling treatment

Slide title
Write your caption hereButton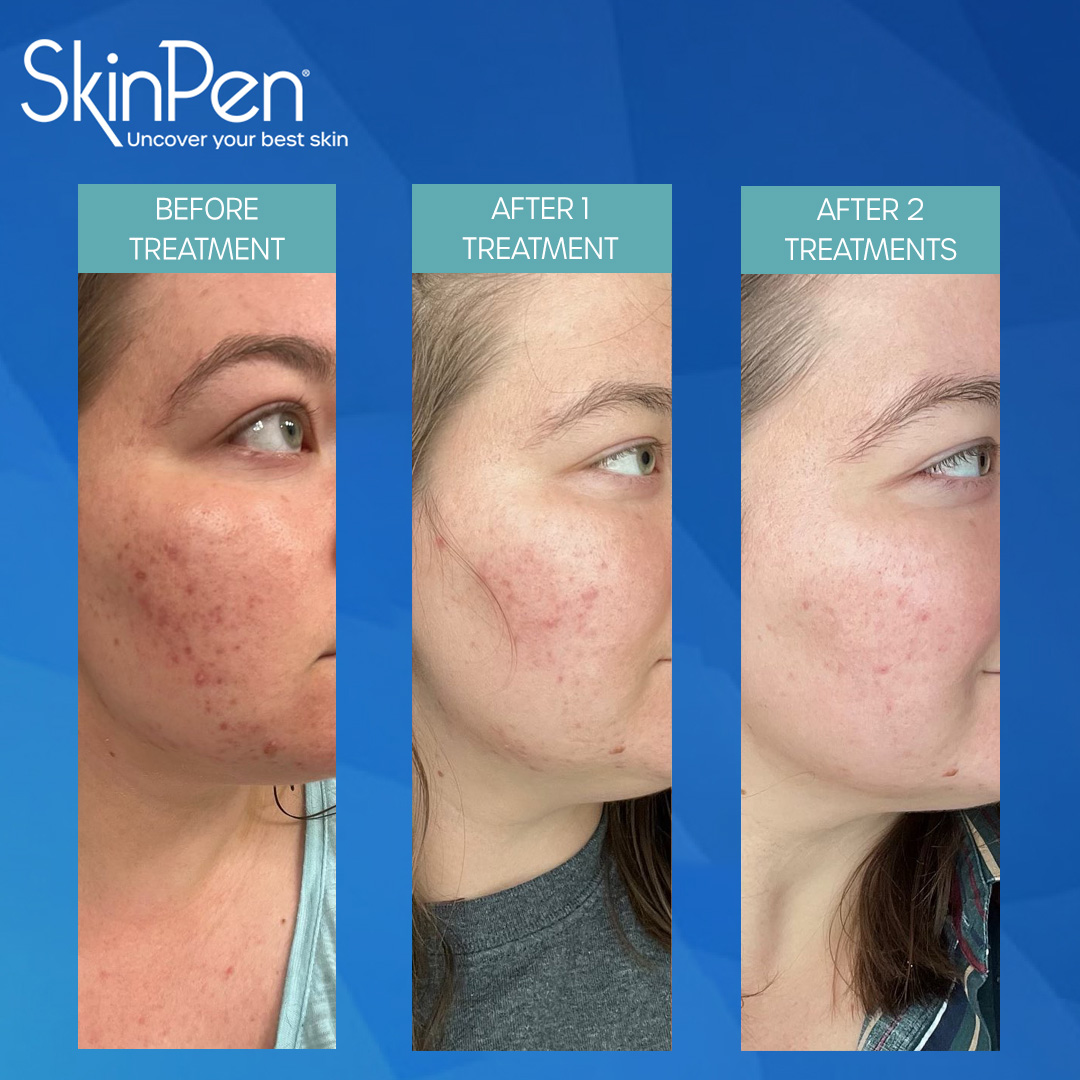
Slide title
Write your caption hereButton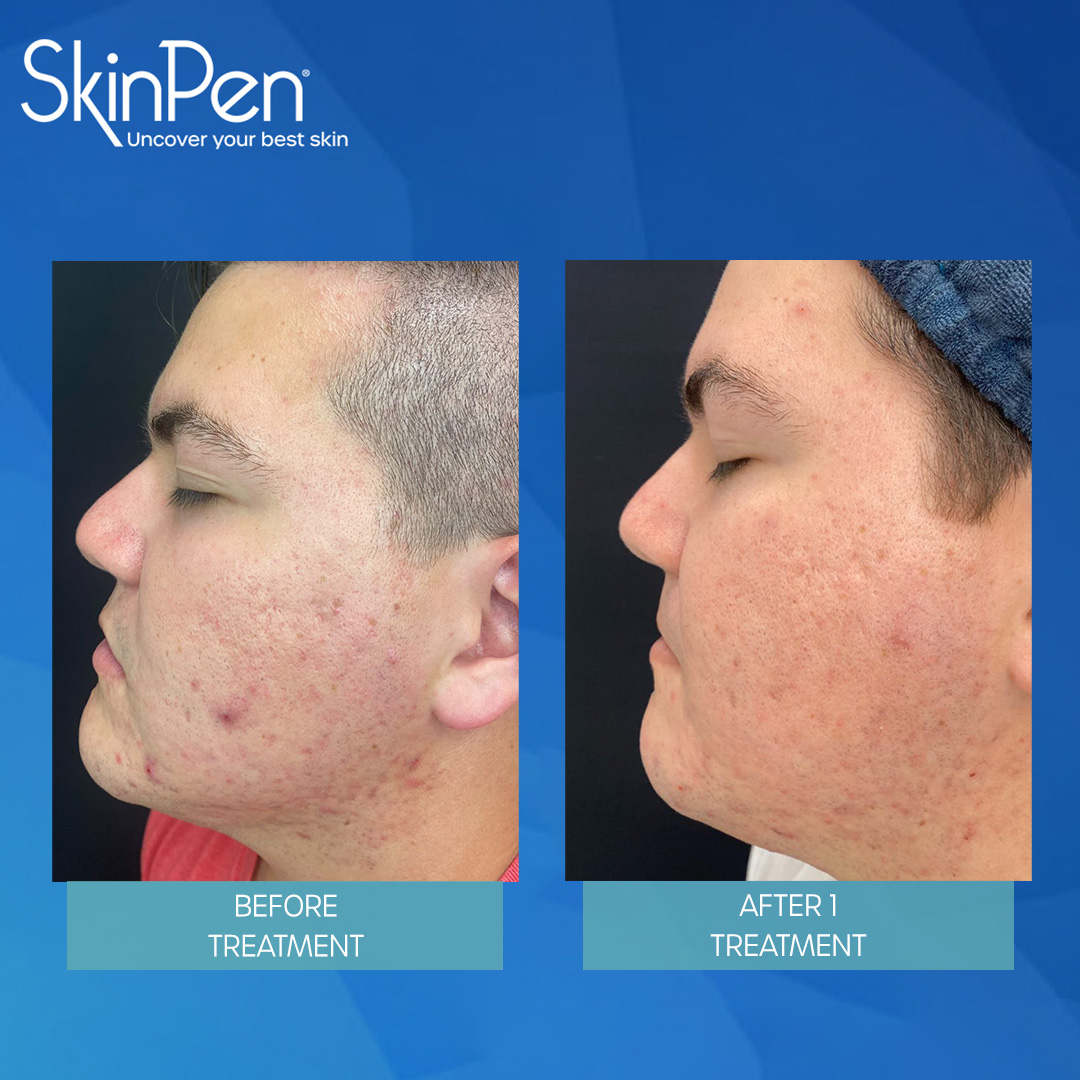
Slide title
Write your caption hereButton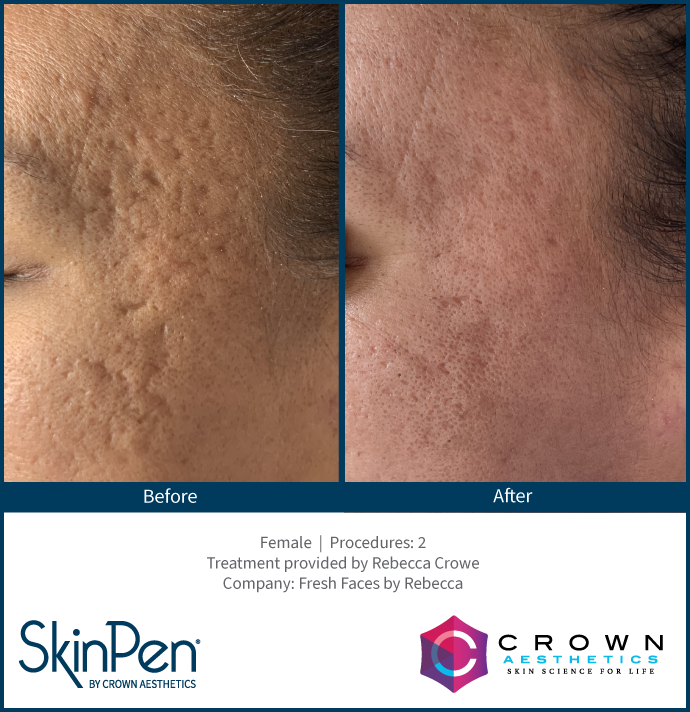
Slide title
Write your caption hereButton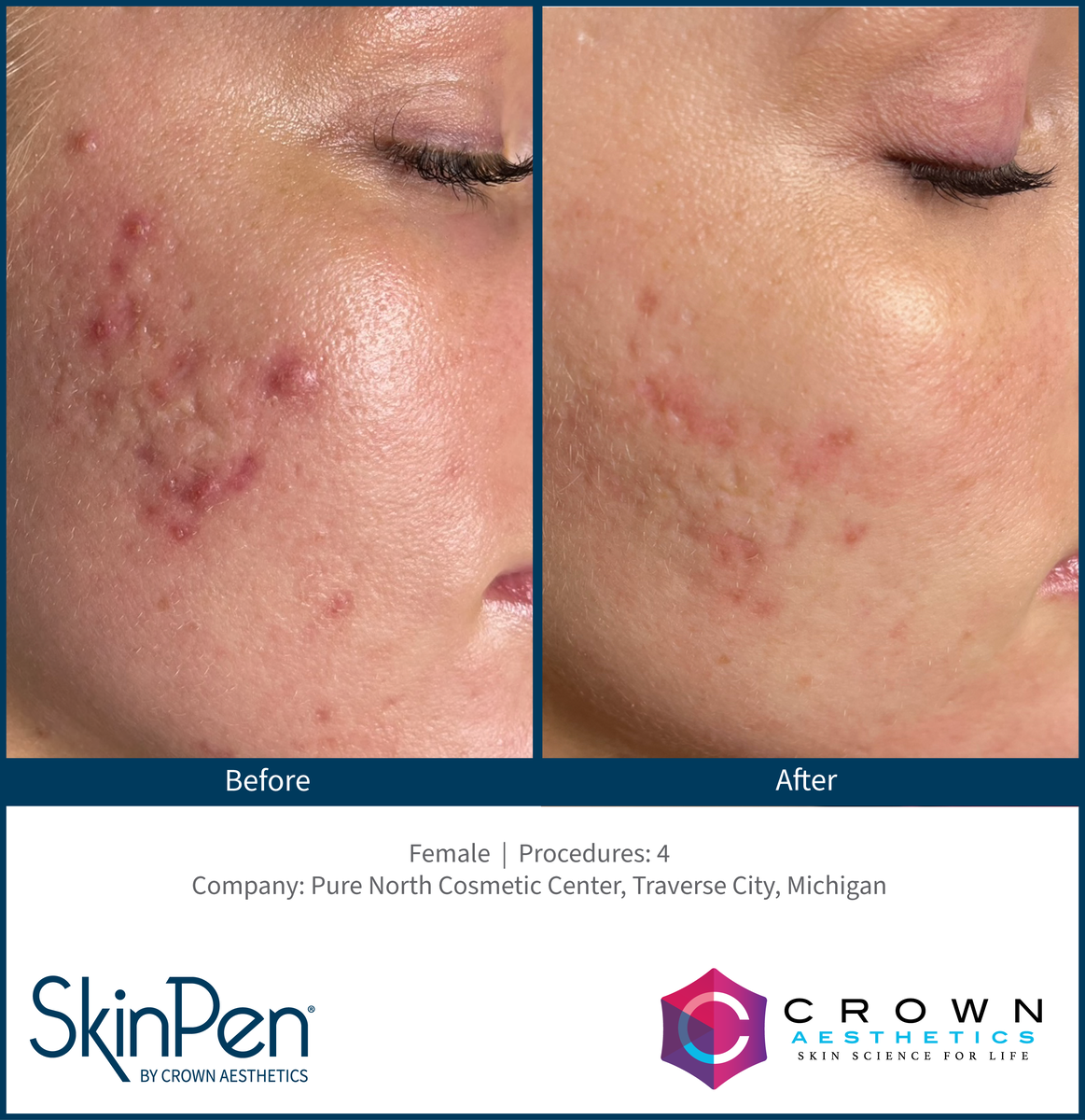
Slide title
Write your caption hereButton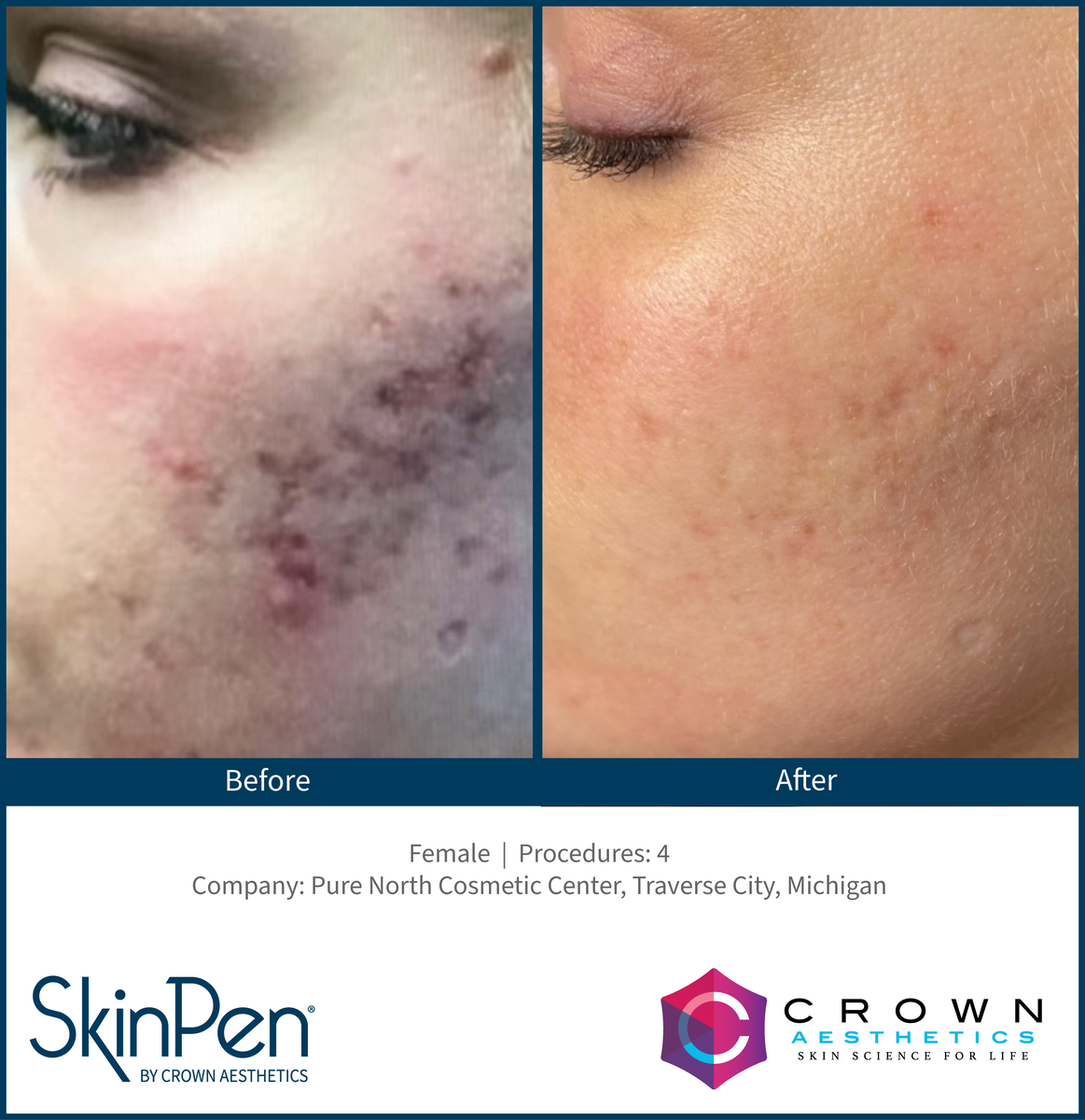
Slide title
Write your caption hereButton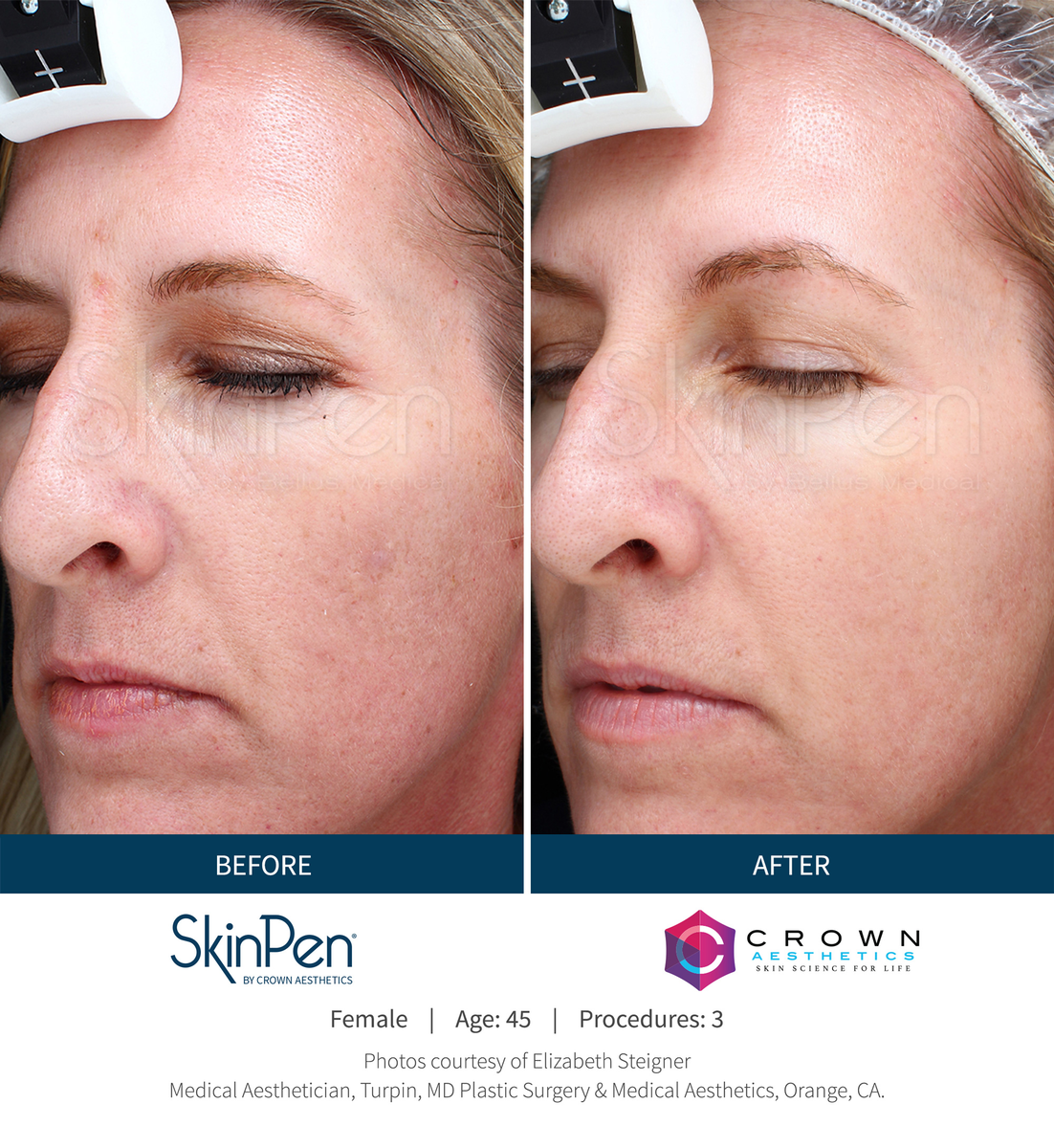
Slide title
Write your caption hereButton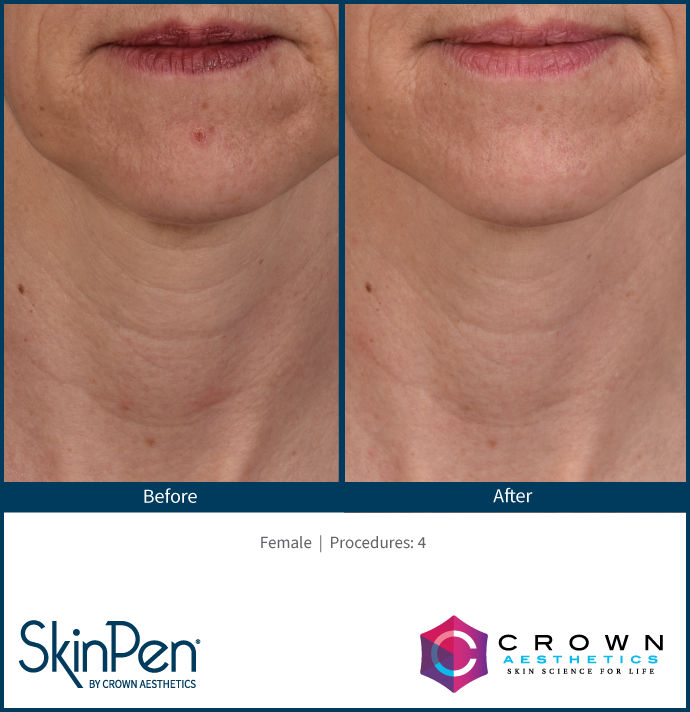
Slide title
Write your caption hereButton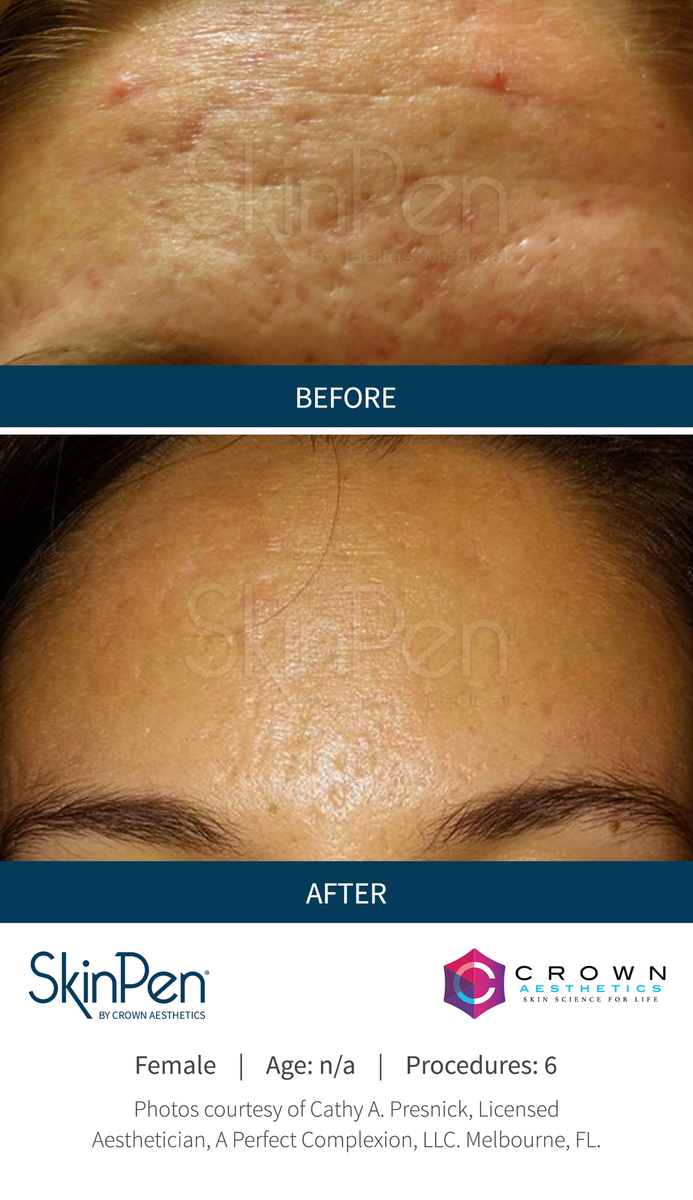
Slide title
Write your caption hereButton
-
IMPORTANT SAFETY INFORMATION - SkinPen®
PRODUCT DESCRIPTION
a. What is it, and how does it work?
SkinPen® Precision System is a prescription only, minimally invasive, micro-needling
device and accessories intended to be used as a treatment to improve the appearance
of wrinkles of the neck for Fitzpatrick skin types II - IV and to improve the appearance
of facial acne scars in adults with all Fitzpatrick skin types aged 22 years and older.
The SkinPen Precision System is comprised of a reusable motor unit designed to
be attached to sterile, disposable cartridges that house 14 micro- needles (~250 um
diameter) on a reciprocating head which when activated and placed properly against
the skin can create hundreds to thousands of “micro” punctures into the skin. The
procedure creates many microscopic punctures in the skin’s outer layers. As these
punctures heal, the remodeling process works to improve the appearance of facial
acne scars and wrinkles on the neck.
SkinPen Precision’s® patented cartridge design inhibits the entrance of fluids into the
device which along with the BioSheath and routine cleaning help to prevent potential
cross-contamination of the motor unit between uses. It is for prescription use only.
b. Who is a candidate?
The SkinPen® Precision system is a microneedling device and accessories intended
to be used as a treatment to improve the appearance of wrinkles of the neck for
Fitzpatrick skin types II - IV and to improve the appearance of facial acne scars in adults
with all Fitzpatrick skin types aged 22 years and older. The SkinPen® Precision System’s
acne scars clinical studies were conducted on adult men and women ranging from light
to dark skin (all Fitzpatrick skin types). The wrinkles clinical study was conducted on
adult men and women with Fitzpatrick skin types II, III and IV.
CONTRAINDICATIONS
a. Are there any reasons why I should not receive a SkinPen® Precision treatment?
The use of the SkinPen® Precision System should not be used on patients who:
a. Have active skin cancer in the treatment area(s)
b. Have open wounds, sores, or irritated skin in the treatment area(s)
c. Have an allergy to stainless steel or anesthetics
d. Have a hemorrhagic (bleeding) disorder or hemostatic (bleeding) dysfunction
e. Are pregnant or nursing
f. Are currently taking drugs with the ingredient isotretinoin (such as Accutane)
NOTE: This product is not intended for transdermal (under the skin) delivery of topical
products such as cosmetics, drugs, or biologics.
PRECAUTIONS
a. What precautions should my doctor advise me about?
The SkinPen® Precision System has not been evaluated in the following patient
populations. Therefore, if you have a history of the following conditions or have
taken the following medications, please let your doctor know, as treatment with the
SkinPen Precision System may not be appropriate for you: Actinic (solar) keratosis;
active acne; collagen vascular diseases or cardiac abnormalities; diabetes; eczema,
psoriasis and other chronic conditions in the treatment area or on other areas of
the body; immunosuppressive therapy; history of contact dermatitis; raised moles in
the treatment area; rosacea; active bacterial or fungal infection, active viral herpes
simplex infections (such as cold sores); warts; keloid scars; patients on anticoagulants
(also known as ‘blood thinners’); scars and stretchmarks less than one year old;
scleroderma; and wound-healing deficiencies. Inform your provider if you have any of
the above conditions. Your doctor will determine whether treatment with the SkinPen
Precision System is right for you.
Let your doctor know if you are allergic or sensitive to any of the following ingredients
which are in the Skinfuse Lift HG hydrogel: purified water, glycerin, carbomer,
potassium hydroxide, disodium EDTA, phenoxyethanol, caprylyl glycol, sorbic acid. If
you are, SkinPen® Precision treatment may not be safe for you.
CLINICAL STUDY – ACNE SCARS
a. How was the product studied?
A clinical study was conducted to support the safety and effectiveness of the SkinPen
Precision System for the treatment of acne scars on the face.
The single center study was conducted on a total of 41 subjects, 20 of which were
exclusive to the SkinPen Precision device (7 male and 13 female), aged 21 years and
older from various ethnic groups with multiple skin tones (pale to dark skin). The other
21 subjects were treated with a prototype device. Treatments were given on day 1, day
30, and day 60, with follow-up visits at 1 month and 6 months after the final (day 60)
treatment. Treatments were conducted by a trained aesthetician (skin care specialist).
The face was cleaned and numbed prior to treatment. A thin layer of Skinfuse Lift
HG was applied prior to treatment to protect against abrasion and friction during
the procedure. The aestheticians were instructed to start at the lowest depth setting
and gradually increase the depth until redness was observed. Following treatment,
Skinfuse Lift HG was applied to prevent the skin from drying out post procedure.
Table 3: Summary of Demographic Information
At each clinical visit, digital images were taken of each subject’s facial acne scars.
These images were graded by two separate Board Certified Dermatologists after
completion of the study using the following assessment tools and timepoints [Table 4].
The dermatologists were blinded, which means they did not know which treatment
or time point was represented in each photo. Details of each of these assessment tools
are provided below in Tables 5-7. The results of the study are provided in Tables 8-12.
Table 4: Study Endpoints
The photo grading included the following effectiveness assessments:
• Acne Scar Assessment Scale1
Table 5: Acne Scar Assessment Scale
In addition to the clinician graded effectiveness measures, the following patientreported measures were recorded throughout the study:
• Self-assessed Scar Improvement Scale
Table 6: Self-assessed Scar Improvement Scale
• Subject Global Aesthetic Improvement Scale
Table 7: Subject Global Aesthetic Improvement Scale
Safety information was collected throughout the study using subject safety diaries.
Safety diaries were provided to the subject at each treatment visit (day 1, 30, and 60). The
subject was instructed to record any observations related to treatment including common
treatment responses. Common treatment responses are side effects that result from
treatment which resolve on the order of days. Common treatment responses that persist
may be categorized as adverse events when assessed by the investigator at the next visit.
Subjects were informed of the following potential common treatment responses in the
informed consent process: skin will be red and flushed similar to a moderate sunburn,
skin tightness and mild sensitivity to the touch, redness, burning, tingling, stinging,
itching, and/or scaling/dryness, edema (swelling), tenderness/ discomfort, a possibility
of developing an infection (an increase in redness, warmth, itching, or pus formation).
The diaries included space for daily recording of observations for the 30 days in between
treatment visits. Adverse events were assessed by the investigator at each subsequent visit.
• Patient Satisfaction Questionnaire
Three questions were asked to the subjects in the study regarding their level of
satisfaction with the treatment. It was included as a secondary endpoint in the study.
See individual questions and results in the section below.
RISKS:
a. What side effects were seen in the clinical study?
Common Treatment Responses:
At the 6-month post-treatment visit, no adverse events were seen.
The following common treatment responses were reported in the subject safety
diaries which were sent home with the subject:
• Dryness in 5/41 (12%) subjects lasting from 1-6 days
o These responses were reported by 3 subjects with FST III, 1 subject with FST VI,
and 1 subject with FST V
• Rough Skin in 3/41 (7%) of subjects lasting from 1-2 days
o These responses were reported by 1 subject with FST III, and 2 subjects
with FST V
• Tightness in 2/41 (4%) of subjects lasting from 1-2 days
o These responses were reported by 2 subjects with FST VI
• Redness, Itching, Peeling Discomfort and Tenderness in 13/41 (31%) of subjects
lasting 1-3 days
o These responses were reported by 6 subjects with FST III, 2 subjects with FST VI, 3
subjects with FST V, and 2 subjects with FST VI
• Burning in 4/41 (9%) of subjects lasting 1-3 days
o These responses were reported by 1 subject with FST III, 1 subject with FST VI,
and 2 subjects with FST V
b. What adverse events were seen in the clinical study?
1 subject reported an insect/bug bite on the inner right thigh that was determined
to be moderate and unlikely related to SkinPen prototype device. 1 subject (1/41,
2.4%) experienced an Adverse Event (skin striae [linear marks, ridges, or grooves] on
the forehead and both sides of the face) that was determined to be mild and possibly
related to use of the SkinPen Precision System. This Adverse Event was thought to be
due to subject exposure to excess sunlight soon after treatment which was against
study instructions, yet resolved without any additional complications.
c. What are other possible adverse events?
Although not seen in the clinical study, patients may experience reactivation of
herpes simplex virus (coldsore), pigment changes that include lighter or darker skin
in the area treatment that resolves over time, or no change in their acne scars.
BENEFITS:
What will a SkinPen Precision Treatment accomplish, and what did the clinical study
show?
The study doctors reported using the Acne Scar Assessment Scale:
Results of the photo grading indicated a significant improvement in acne scar
assessment at 6 months post-treatment when compared with baseline with 55%
(11/20) of subjects showing improvement at 6-months post-treatment when
compared with baseline. At 6-months post-treatment, the remaining 9 subjects(45%)
reported no change in score when compared to baseline. The visual improvements
seen in the photo grading results were considered to be clinically meaningful.
Table 8: Results of Photo Grading of Acne Scar Assessment Scale for SkinPen
Precision System
Table 9: Change from Baseline for Photo Grading of Acne Scar Assessment Scale for
SkinPen Precision System
Subjects reported using the Self-assessed Scar Improvement Scale:
Treatment with SkinPen Precision produced an improvement in SASIS scores at
1-month post-treatment and 6-months post-treatment. At 1-month post-treatment,
17 (85%) subjects reported some percentage of improvement in the appearance of
their acne scars, with 3 (15%) subjects reporting no change.
At 6-months post-treatment, 18 (90%) subjects reported some percentage of
improvement in the appearance of their acne scars, with 2 (10%) subjects reporting
no change. The average value for the population was = 1.65 and 1.70, at 1-month
post-treatment and 6-months post-treatment respectively (1%-25% improvement in
appearance of acne scars) when compared with a score of 0 (no change in appearance
of acne scars). No subjects reported a negative score (i.e., worsening of acne scars) at
either post-treatment timepoint.
Subjects reported using the Subject Global Aesthetic Improvement Scale:
Treatment with SkinPen Precision produced an improvement in SGAIS scores at 1
month post-treatment and 6 months post-treatment. At 1-month post-treatment,
7 (35%) subjects reported much improved, 9 (45%) subjects reported improved,
and 4 (20%) subjects reported no change. At 6-months post- treatment, 2 (10%)
subjects reported very much improved, 8 (40%) subjects reported much improved,
8 (40%) subjects reported improved, and 2 (10%) subjects reported no change. The
mean value for the population was = 2.85 and 2.50, at 1-month post-treatment and
6-months post-treatment improved when compared with a score of 4 (no change). No
subjects reported a score of 5 (worse) at either post treatment timepoint.
Subjects reported using the Patient Satisfaction Questionnaire:
The results of the patient satisfaction questionnaire for all subjects indicated that
a greater number of subjects (11/20, 55%) selected favorable responses regarding
treatments at 1 month and 6 months post-treatment for the following inquiries:
SkinPen Precision System All Subjects
N 20 41
Age (years)
Mean (standard deviation) 43.8 (12.7) 44 (11.9)
Minimum, Median, Maximum 23, 48, 60 21, 46, 60
N (%) N (%)
Sex
Male 7 35 13 31.7
Female 13 65 28 68.3
Ethnicity
Hispanic or Latino 6 30 13 31.7
Not Hispanic or Latino 14 70 28 68.3
Race
American Indian or Alaska Native 1 5 2 4.9
Asian 3 15 9 22.0
Black or African American 6 30 10 24.4
White 10 50 20 48.8
Fitzpatrick Skin Type
II 2 10 3 7.3
III 4 20 10 24.4
IV 7 35 17 41.5
V 4 20 7 17.1
VI 3 15 4 9.8
Primary
effectiveness
endpoints
Acne Scar Assessment Scale graded by two blinded dermatologists using
photographs taken at baseline, day 30, day 60, 1-month post-treatment, and
6-months post-treatment
Clinician’s Global Aesthetic Improvement Assessment graded by two blinded
dermatologists using photographs taken at 1-month post-treatment, and
6-months post-treatment
Secondary
effectiveness
endpoints
Self-assessed Scar Improvement Scale completed by subjects at baseline,
1-month post-treatment, and 6-months post-treatment
Subject Global Aesthetic Improvement Scale completed by subjects at baseline,
1-month post-treatment, and 6-months post-treatment
Patient Satisfaction Questionnaire completed by subjects at 1-month posttreatment and 6-months post-treatment
Safety
Endpoint
Subject safety diaries provided to the subject at each treatment visit (day 1, 30,
and 60) and completed for 30 days to record treatment responses
Adverse event monitoring at each visit; baseline, day 30, day 60, 1-month posttreatment, and 6-months post-treatment
Grade Term Description
0 Clear No depressions are seen in the treatment area. Macular discoloration
may be seen.
1 Very mild A single depression is easily noticeable with direct lighting (deep).
Most or all of the depressions seen are only readily apparent with
tangential lighting (shallow).
2 Mild A few to several, but less than half of all the depressions are easily
noticeable with direct lighting (deep). Most of the depressions seen
are only readily apparent with tangential lighting (shallow).
3 Moderate More than half of the depressions are apparent with direct lighting
(deep).
4 Severe All or almost all the lesions can be seen with direct lighting (deep).
1
Jwala Karnik, Leslie Baumann, Suzanne Bruce, Valerie Callender, Steven Cohen, Pearl Grimes, John Joseph,
Ava Shamban, James Spencer, Ruth Tedaldi, William Philip Werschler, Stacy R. Smith, “A double-blind,
randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the
correction of atrophic facial acne scars” Journal of the American Academy of Dermatology 71(1):77-83 (2014).
Rating Description
-1 Exacerbation of Acne Scars
0 No change in appearance of acne scars
1 1% - 25% improvement in appearance of acne scars
2 25% - 50% improvement in appearance of acne scars
3 50% - 75% improvement in appearance of acne scars
4 75% - 99% improvement in appearance of acne scars
4 All or almost all the lesions can be seen with direct lighting (deep).
Rating Description
1 Very Much Improved: Optimal cosmetic result.
2 Much Improved: Marked improvement in appearance from the initial condition,
but not completely optimal.
3 Improved: Obvious improvement in appearance from initial condition.
4 No Change: The appearance is essentially the same as the original condition.
5 Worse: The appearance is worse than the original condition.
Time Point N Mean Standard
Deviation
Min Median Max
Baseline 20 2.80 0.52 2.00 3.00 4.00
Day 30 20 2.78 0.57 2.00 2.75 4.00
Day 60 20 2.70 0.55 2.00 2.50 3.50
1-Month Post- Treatment 20 2.68 0.49 2.00 2.50 3.50
6-Months Post- Treatment 20 2.35 0.69 1.50 2.50 3.50
Time Point N Subject Improved (%) Subject Worsened (%)
Day 30 20 30.0 20.0
Day 60 20 35.0 20.0
1-Month Post-Treatment 20 40.0 20.0
6-Months Post-Treatment 20 55.0 0.0
(Continued on reverse side.)
• Question 1: Do you notice any improvement in how your acne scars look in the
treated area?
Table 10: Results of Patient Satisfaction Questionnaire - Question 1
• Question 2: How would you characterize your satisfaction with the treatment?
Table 11: Results of Patient Satisfaction Questionnaire – Question 2
• Question 3: Would you recommend this treatment to your friends and family
members?
Table 12: Results of Patient Satisfaction Questionnaire – Question 3
CLINICAL STUDY – WRINKLES
a. How was the product studied?
A clinical study was conducted to support the safety and effectiveness of the SkinPen
Precision System for the treatment of wrinkles on the neck.
The single center study was conducted on a total of 35 subjects (2 male and 33 female),
aged 44 years and older from various ethnic groups with multiple skin tones (pale to
dark skin). Treatments were given on day 1, day 30, day 60, and day 90 with follow-up
visits at 1 month and 3 months after the last treatment. Under direct supervision of a
licensed Physician, treatments were conducted by a trained aesthetician (skin care
specialist). The face and neck was cleaned and numbed prior to treatment. A thin layer
of Skinfuse Lift HG was applied prior to treatment area to protect against abrasion and
friction during the procedure. The aestheticians were instructed to treat at depths of
up to 2.5 mm. Following treatment, Skinfuse Lift HG was applied to prevent the skin
from drying out post procedure.
Table 13: Summary of Demographic Information Per Protocol
At each clinical visit, digital images were taken of each subject’s wrinkles on the neck.
These images were graded by two separate independent blinded Board Certified
Physicians after completion of the study using the following assessment tools and
timepoints [Tables 14-17]. The results of the study are provided in Tables 18-22.
Table 14: Study Endpoints
Subjects had wrinkling assessed on the neck using the G. Lemperle Wrinkle
Assessment Scale.
Table 15: Assessment of Wrinkling – G. Lemperle Wrinkle Scale
At 1 month and 3 months post-treatment, subjects also participated in the following
procedures:
• Clinician’s Global Aesthetic Improvement Scale
Table 16: Clinician’s Global Aesthetic Improvement Scale (CGAIS)
• Subject’s Global Aesthetic Improvement Scale
Table 17: Subject Global Aesthetic Improvement Scale
• Patient Satisfaction Questionnaire
Subjects completed a Sponsor-provided questionnaire regarding improvement
in wrinkles, satisfaction with the treatment and willingness to recommend the
treatment to friends and family members
RISKS:
a. What side effects were seen in the clinical study?
Common Treatment Responses on the face and neck:
Common Treatment responses of dryness, redness, burning sensation and itchiness
which lasted the duration of 1-3 days. Reactions of tenderness and peeling/flaking
occurred for the duration of 1-7 days.
The following common treatment responses were reported in the subject safety
diaries which were sent home with the subject:
• Dryness in 7/32 (22%) subjects lasting from 1-3 days
o These responses were reported by 6 subjects with FST II, 1 subject with FST IV
• Redness in 2/32 (6%) subjects lasting from 1-3 days
o These responses were reported by 2 subjects, 1 subject with FST II, 1 subject with
FST IV
• Itching in 1/32 (3%) subjects with FST II, lasting from 1-2 days
• Peeling was reported in 8/32 (22%) of subjects lasting 1-3 days
o These responses were reported by 8 subjects, 5 subjects with FST II, 1 subject
with FST III, 2 subjects with FST IV
• Tenderness that lasted 1-4 days in 1/32 (3%) of Subjects , with FST II
• Burning in 2/32 (6%) of subjects lasting 1-3 days
o These responses were reported by 2 subjects with FST IV
b. What adverse events were seen in the clinical study?
At the 3-month post-treatment visit, no adverse events were seen.
No adverse events related to the SkinPen Precision treatment were observed on the
face or neck during the study.
c. What are other possible adverse events?
Although not seen in the clinical study, patients may experience red and flushed
skin, skin tightness and mild sensitivity to touch (such as itching, burning, stinging,
tingling), scaling/dryness, redness, edema and tenderness/discomfort.
BENEFITS:
What will a SkinPen Precision Treatment accomplish, and what did the clinical study
show?
The study doctors reported using the G. Lemperle Wrinkle Scale:
Results of the photo grading indicated a significant improvement in wrinkles on the
neck area assessment at 3 months post-treatment.
Table 18: Results of Photo Grading of G. Lemperle Wrinkle Scale for SkinPen Precision
System
Table 19: Change from Baseline for Photo Grading of G. Lemperle Wrinkle Scale for
SkinPen Precision System
Clinician’s Global Aesthetic Improvement Assessment:
Treatment with SkinPen Precision produced an improvement in CGAIS scores at
3 months post-treatment. At three-months post-treatment evaluation, 31.5% of
subjects received a ‘3: improved’ grading and 57% received a grading of ‘4: no change’
relative to pre-treatment. Four subjects (11.5%) received a grading of ‘2: much
improved’.
Subjects reported using the Subject Global Aesthetic Improvement Scale:
Treatment with SkinPen Precision produced an improvement in Subject GAIS scores
at 3-months post-treatment. At 3-months post-treatment, 22 (68.8%) subjects
reported some percentage of improvement in the appearance of their wrinkles, with
10 (31.3%) subjects reporting no change.
Subjects reported using the Patient Satisfaction Questionnaire:
The results of the patient satisfaction questionnaire for all subjects indicated that a
greater number of subjects selected favorable responses regarding treatments at 1
month and 3 months post-treatment for the following inquiries:
• Question 1: Do you notice any improvement in how your fine lines and wrinkles look
in the treated area?
Table 20: Results of Patient Satisfaction Questionnaire - Question 1
• Question 2: How would you characterize your satisfaction with the treatment?
Table 21: Results of Patient Satisfaction Questionnaire – Question 2
• Question 3: Would you recommend this treatment to your friends and family
members?
Table 22: Results of Patient Satisfaction Questionnaire – Question 3
Subjects were informed of the following potential common treatment responses
in the informed consent process: skin will be red and flushed similar to a moderate
sunburn, skin tightness and mild sensitivity to the touch, redness, burning, tingling,
stinging, itching, and/or scaling/dryness, edema (swelling), tenderness/ discomfort, a
possibility of developing an infection (an increase in redness, warmth, itching, or pus
formation). The diaries included space for daily recording of observations for the 30
days in between treatment visits. Adverse events were assessed by the investigator at
each subsequent visit.
BEFORE TREATMENT INFORMATION
a. What happens in the office before the SkinPen® Precision treatment?
Note that each doctor may have a unique process for assessing and treating patients.
The following is an example of what you would likely experience with a typical
SkinPen® Precision treatment. Before the SkinPen® Precision treatment, your doctor
will ask you questions about your medical history, as well as your treatment goals. Your
doctor will discuss whether you are an appropriate candidate for a SkinPen® Precision
System treatment and review what to expect during and after a treatment, including
common treatment responses and adverse events.
You may be cautioned to avoid sun exposure and stop topical retinoid therapy 24 hours
prior to procedure. You should allow at least 24 hours after autoimmune therapies
before a treatment. You should wait six months following oral isotretinoin (such as
Accutane) use before receiving treatment.
Your doctor will also examine your treatment area, and may take photos. Different
options for pain management will be discussed, and if pretreatment numbing is
desired, a topical anesthetic agent may be used. The treatment area will be cleaned
and then prepared with isopropyl alcohol or other antiseptic before the treatment.
TREATMENT DESCRIPTION
a. What happens during the treatment?
A layer of Skinfuse Lift HG is applied to the treatment area to protect the skin against
abrasion and friction of the SkinPen® Precision device during treatment.
NOTE: Let your doctor know if you are allergic or sensitive to any of the following
ingredients which are in the Skinfuse Lift HG hydrogel: purified water, glycerin,
carbomer, potassium hydroxide, disodium EDTA, phenoxyethanol, caprylyl glycol,
sorbic acid. If you are, the SkinPen® Precision treatment may not be safe for you. The
doctor then selects a depth on the microneedle cartridge to begin treatment on the
patient and begins the treatment. As the SkinPen® Precision glides over the skin microchannels are created on the skins surface. Gauze may be used to pat down the affected
area after treatment, and it is suggested to apply a generous layer of Skinfuse Lift HG to
prevent the skin from drying out post treatment.
b. Does microneedling with the SkinPen® Precision hurt?
The SkinPen® Precision treatment may cause some minor discomfort during after the
treatment. Your doctor may recommend that a topical numbing agent be used during
treatment to further minimize discomfort.
AFTER TREATMENT INFORMATION
a. What should I expect following the treatment?
Possible treatment responses include dryness, rough skin, tightness, redness, itching,
peeling, discomfort, tenderness, and burning. These conditions will resolve over time
without any further complications.
Although not seen in the clinical study for acne scars, you may experience reactivation
of herpes simplex virus (cold sore), pigment changes that include lighter or darker skin
in the area treatment that resolves over time, or no change in their acne scars.
Your doctor will also tell you what to expect following a SkinPen® Precision system
treatment. Within the first 72 hours post-treatment you should avoid sweaty exercise
and sun exposure. Exposure to these conditions could lead to: itching, burning,
stinging, and tingling), scaling/dryness, redness, swelling, and tenderness/discomfort.
b. Will I need more than one treatment to achieve my desired results for treatment of
acne scars?
You should discuss treatment goals with your doctor. In the clinical study patients
were treated in a series of 3 treatments spaced 4 weeks apart. It is recommended to
avoid other facial aesthetic treatments the month following your SkinPen Precision
treatment.
c. Will I need more than one treatment to achieve my desired results for treatment of
wrinkles on the neck?
You should discuss treatment goals with your doctor. In the clinical study patients
were treated in a series of 4 treatments spaced 4 weeks apart. It is recommended to
avoid other facial aesthetic treatments the month following your SkinPen Precision
treatment.
d. Do the results last forever?
Acne Scars: While individual results may vary, in the clinical study, the results were
evaluated 6 months after the third (final) treatment. After this, additional treatments
may be needed to maintain your desired result.
Wrinkles on the Neck: While individual results may vary, in the clinical study,
the results were evaluated 3 months after the fourth (final) treatment. After this,
additional treatments may be needed to maintain your desired result.
WHEN TO CALL YOUR DOCTOR?
a. When should I call my doctor?
Call your doctor immediately if you experience:
1. Signs of infection such as an increase in redness, warmth, itching, or pus formation.
This would typically happen within a day or two after the treatment.
2. Allergic reactions to the test material (s). Rare allergic reactions can consist of severe
contact dermatitis (rash) or itching, swelling, or difficulty breathing. This risk is
increased for individuals with a history of allergies, and individuals with asthma
and/or a history of hives, and itching.
Be sure to call your doctor if you:
3. Any additional treatment responses not discussed above.
ADDITIONAL INFORMATION
a. What should I do if I have additional questions?
For further questions and information, please call Crown Aesthetics at 1.888.372.3982.
Address: 5005 Lyndon B. Johnson Fwy, Suite 370, Dallas, TX 75244.
GLOSSARY OF TERMS:
Actinic keratosis – A rough, scaly patch on your skin that develops from years of exposure
to the sun
Active acne – Acne where there are currently pustules, cysts or lesions on the skin at the time
of evaluation
Adverse Event – A negative reaction to a medication or treatment
Anesthetic – A substance that induces insensitivity to pain, a numbing agent.
Anticoagulant – Blood thinner
Cardiac abnormality – Abnormal heart/blood vessel structure or function
Contact dermatitis – A rash that occurs at the site of exposure to a substance capable of
producing an irritant skin response.
Diabetes – A disease in which your blood glucose, or blood sugar, levels are too high
Eczema – A condition that makes your skin red and itch
Fitzpatrick Skin Types – A numerical classification system for human skin color
Hemorrhagic – Accompanied by or produced by an escape of blood from a ruptured blood
vessel, especially when bleeding excessively
Hemostatic – The stopping of blood flow
Immunosuppressive – Partially or completely suppressing the immune response of an
individual
Isotretinoin – Vitamin A derivative that is used to treat acne. Common brand names:
Accutane, Myorisan
Keloid scars – A scar that grows outside the boundaries of the original scar.
Orbital rim – The portion of the skull that outlines the border of the eye socket
Papules – A small bump (up to 1 cm in diameter) on the skin with no visible fluid within
Psoriasis – a common skin condition that causes cells to build up rapidly on the surface of
the skin to form thick, silvery scales and itchy, dry, red patches that are sometimes painful
Rosacea – A disease that affects your skin and sometimes your eyes. It causes redness and
pimples.
Scleroderma – A number of rare diseases that involve the hardening and tightening of the
skin and connective tissues
Sequelae – Any complications that follow a medical illness or event
Side effect – An expected reaction to a medication or treatment that is used in an approved
manner
Skin striae – A linear mark, slight ridge, or groove
Transdermal – Under the skin
Vesicle – A small blister on the skin
